Health Canada is advising Canadians that more hand sanitizers are being recalled from the marketplace because they may pose health risks.
The federal agency states that they either contain ingredients that are not permitted by Health Canada or are not properly labelled and are missing important information.
In some instances, these sanitizers contain industrial-grade DA-2I ethanol, which is not authorized for use in hand sanitizers.
Consumers are advised to immediately stop using the affected products and consult a healthcare professional if they have used any of these products and have health concerns.
Health Canada maintains this list of hand sanitizers that may pose health risks, so that Canadians can easily identify products they may have purchased and take appropriate action. Canadians are encouraged to check it regularly for updates.
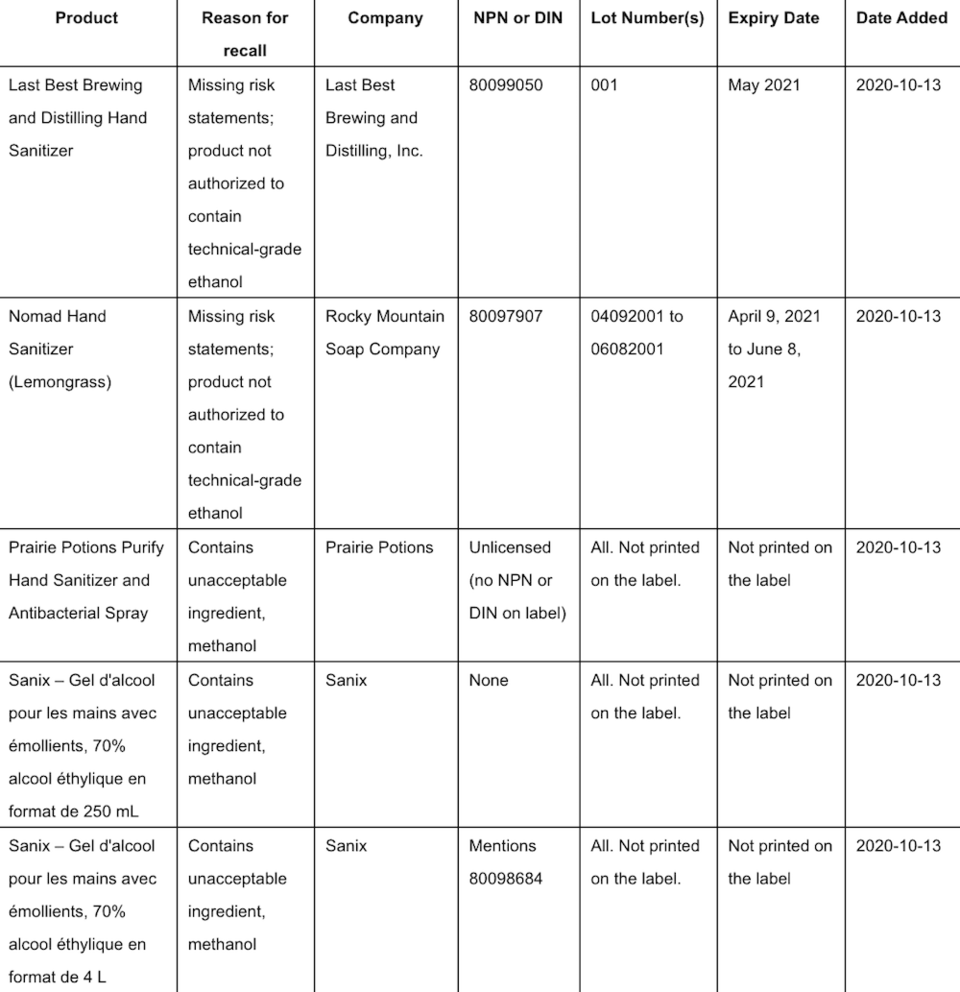 Photo: Health Canada
Photo: Health CanadaThe COVID-19 outbreak has created a high demand for hand sanitizers. To increase the supply, Health Canada has taken several measures, including permitting the temporary use of technical-grade ethanol in alcohol-based hand sanitizers.
Manufacturers wishing to use technical-grade ethanol must choose from a list of Health Canada-authorized suppliers. They must receive a No Objection Letter from us before they can manufacture or distribute the product.
Technical-grade ethanol contains more impurities than pharmaceutical- and food-grade ethanol. Therefore, manufacturers must include the following risk statements on their product labels:
- Under Medicinal Ingredients: "Ethanol XX% (technical-grade)"
- Under Directions: "Adults only"
- Under Warnings: "Do not use on broken or damaged skin," "Not recommended if you are pregnant or breastfeeding" and "Do not inhale"
- Include a statement for consumers: "Report any incident to Health Canada"
- Under Questions: "Call 1-866-234-2345 to report any adverse reaction"
Hand sanitizers that contain unacceptable grades of ethanol or denaturants that are not approved for sale in Canada have not been reviewed for safety or efficacy. Denaturants are added to ethanol to make it taste bad, to discourage the unintentional ingestion of hand sanitizers, especially by children.
Two unauthorized denaturants have been found in hand sanitizers sold in Canada:
- Ethyl acetate: Frequent use of hand sanitizer containing ethyl acetate may cause dry skin, leading to irritation or cracking
- Methanol: Frequent use of hand sanitizer containing methanol may cause dermatitis, eye irritation, upper respiratory system irritation and headaches
A list of affected products can be found HERE. Health Canada says that it will update this list if it becomes aware of other affected products.
Canadians are encouraged to consult the list regularly for updates.
What you should do
- Stop using the products. Please follow municipal or regional guidelines on how to dispose of chemicals and other hazardous waste. You may also return the product to your local pharmacy for proper disposal.
- Consult your healthcare professional if you have used these products and have health concerns.
- To help limit the spread of COVID-19, wash your hands often with soap and water for at least 20 seconds. Use alcohol-based hand sanitizers if soap and water are not available.
- Use hand sanitizers that have been authorized for sale in Canada. Hand sanitizers that have been authorized will display either a Natural Product Number (NPN) or Drug Identification Number (DIN) on the product label.
- Consult the list of hand sanitizers authorized or registered in other jurisdictions that may not display an NPN or DIN but have been accepted for use in Canada during the COVID-19 pandemic. This list of accepted products can be found here.
- Report any health product adverse events or complaints to Health Canada.
Report any health product adverse events or complaints to Health Canada.



